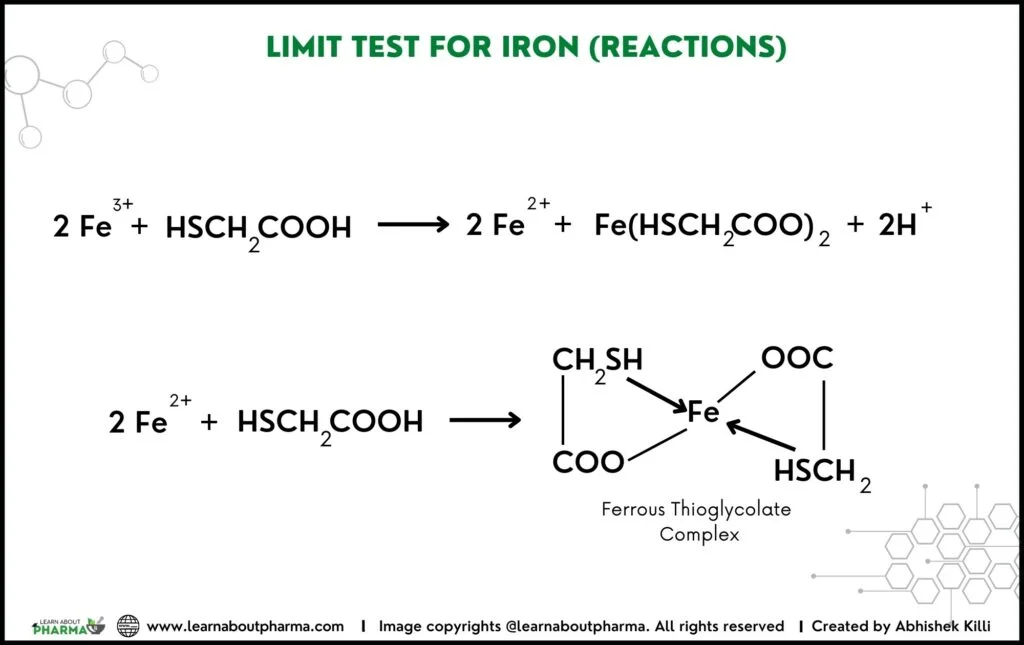
Principle of Iron Limit test: The limit test for Iron is based on the reaction of Iron in ammonical solution with thioglycolic acid in the presence of citric acid to form iron thioglycolate. This reaction turns the pale pink colour of iron thioglycolate into reddish-purple colour.
- The colour is due to the formation coordination compound, ferrous thioglycollate, which is stable in the absence of air and fades in the air due to oxidation. Therefore, the colour should be compared immediately after the time allowed for the full development of colour is over.
- Ferrous thioglycolate is colourless in an acidic medium, but in an alkaline medium, it gives purple colour. The original state of Iron is immaterial, as thioglycolic acid reduces ferric (Fe3+) to ferrous (Fe2+) form.
- Citric acid forms a soluble complex with Iron and prevents its precipitation by ammonia as ferrous hydroxide. Other metal cations’ interference is eliminated by using citric acid, which creates a complex with other metal cations.
You might want to read these:
Aim: This experiment aims to carry out the limit tests for Iron in the given samples.
Apparatus: Measuring cylinder, glass rod, pipette and Nessler’s cylinder
Chemicals: Thioglycolic acid, citric acid, Ammonia solution, ferric ammonium sulphate.
Procedure for limit test for Iron: A standard and test solution is required to perform a limit test for Iron. Let us understand the preparation of these solutions. Take two Nessler’s cylinders and name one as “test” and the other as “standard”.
Preparation of Test solution:
- Accurately measure the known amount of sample as directed in the pharmacopoeia and dissolve it in a specific amount of distilled water. Finally, make up the volume to 40ml using distilled water.
- To this solution, add 2ml of 20%w/v of citric acid (Iron free) and 2 drops of thioglycolic acid and make the solution alkaline by adding ammonia. Finally, adjust the volume to 50ml using distilled water.
- Stir immediately with a glass rod and allow standing for 5 minutes.
Preparation of Standard solution:
- Add 2ml of the standard lead solution to the Standard bottle, add 40 mL of distilled water and mix it thoroughly.
- To the above solution, add 2ml of 20%w/v of citric acid (Iron free) and 2 drops of thioglycolic acid and make the solution alkaline by adding ammonia. Finally, adjust the volume to 50ml using distilled water.
- Stir immediately with a glass rod and allow standing for 5 minutes
Observation:
After standing for 5 min, examine the Nessler’s cylinders laterally against a black background and observe side by side. The intensity of the purple colour produced in the sample solution should not be greater than that of the standard solution. If the intensity of the purple colour in the sample solution is less than the standard solution, then the sample is said to pass the limit test for Iron and vice versa.
Note for limit test for Iron:
- Standard Iron Solution: Weigh 0.1726 g of ferric ammonium sulphate accurately and dissolve in 10 mL of 0.1 N sulphuric acid and sufficient distilled water to produce 1 Litre. Each mL of this solution contains 0.02 mg of Fe.
- Earlier, ammonium thiocyanate reagent was used for the limit test of Iron. Since thioglycolic acid is a more sensitive reagent, it has replaced ammonium thiocyanate in the test.



