There is a saying in the healthcare industry that “if it’s undocumented, it didn’t happen.” Those working in the healthcare sector must have a good understanding of all good documentation practices (GDP) to ensure data integrity and compliance with the regulatory requirements.
Definition:
Good Documentation Practice (GDP): It is the most commonly used term within the pharmaceutical and medical device industries, which describes standards for the preparation, review, approval, issue, record, storing, and archive of any document throughout its lifecycle.
Principle:
ALCOA forms the cornerstone and guiding principles for data integrity and Good Documentation Practices (GDP). It stands for Attributable, Legible, Original, Contemporaneous, Accurate, Complete. They cover the fundamentals of developing your documentation system per the regulatory requirements. They also serve as a base framework for handling data in Good Manufacturing Practices (GMP) and Good Documentation Practices (GDP). We have written a detailed article on ALCOA with a few practical examples to give you a thorough understanding of the concept. Click here to read the article.
Good documentation practices – Guidance for Document Preparation
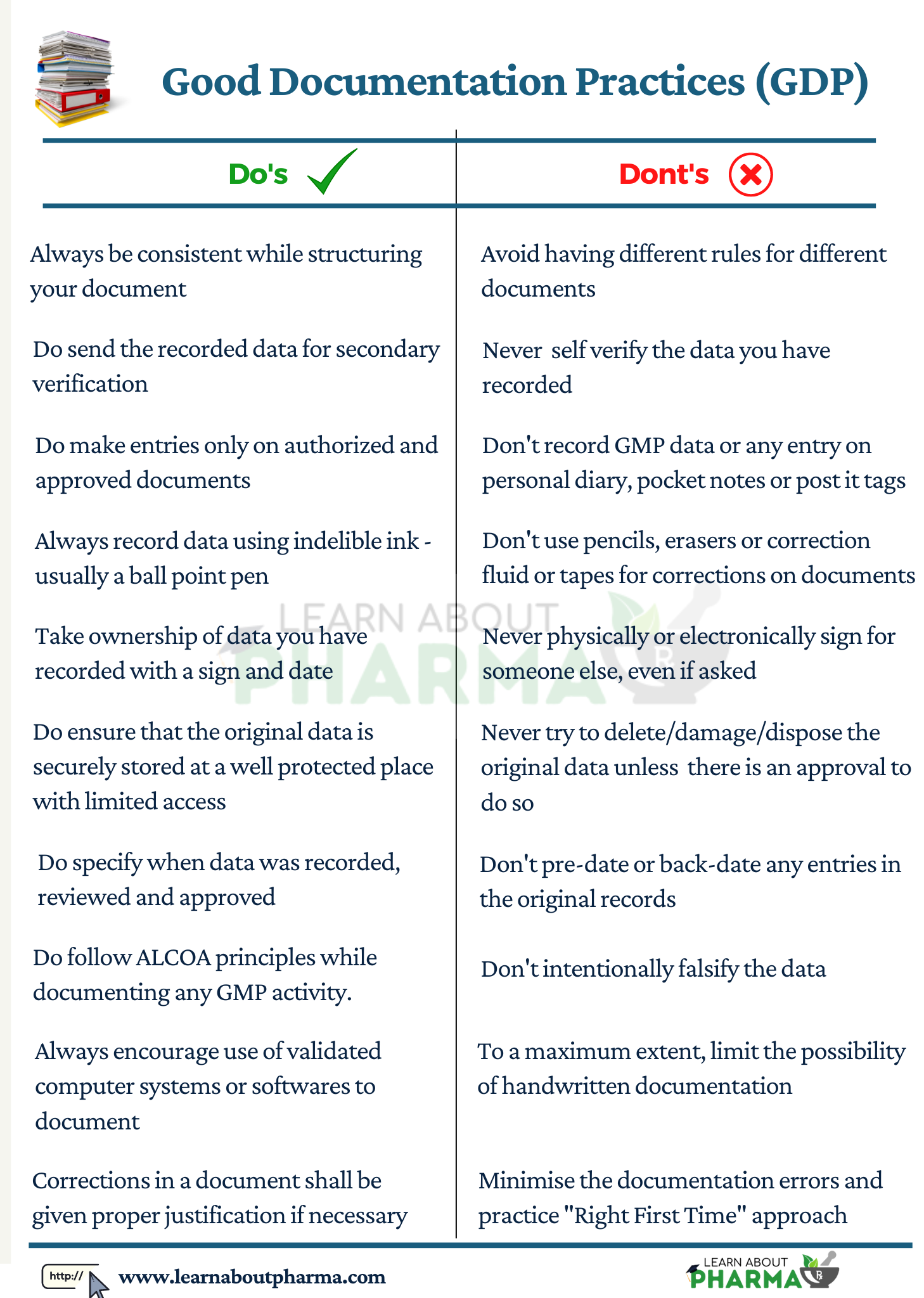
- Documents shall have clear and concise information. All documents must be accurate and written in a manner that prevents errors and ensures consistency
- Based on company requirements, all documents shall preferably be printed on white paper of uniform size (A4, A3, or letter) in a prescribed format.
- Any abbreviations, acronyms, and symbols used shall be explained in complete form in the document. For Example, Abbreviations: e.g. (Example); Acronyms: GMP (Good Manufacturing Practices); in case of symbols: ℃ (Degree centigrade)
- The page numbering of each document shall be in the format of Page XX of YY or any formats decided appropriate by the QA department.
- All documents shall be written in English in an easy-to-understand manner.
- Whenever necessary, a few regions demographically dominated by people with local languages like Hindi, Spanish, French, etc., shall have all documents translated into that language.
- Each document shall contain a reference number of the parent document from which it is generated for easy tracking and monitoring in case of revision wherever applicable.
- Each document shall contain an effective date and revision number wherever applicable.
- Each document shall clearly state the responsibility of persons who prepares, approves, and authorizes the document as applicable.
- All documents shall contain the company’s name and logo with the Signatures and dates of the personnel involved in preparation, Approval, and Authorization as applicable. (Refer to below figure: Format for good document).

- Documents and records should be reviewed by someone who did not perform the task to ensure that the information is correct and accurate. Unsigned documents or records are incomplete and should not be used to perform any task or considered as evidence of a completed task
- All the information shall be written in a manner that is easy to read and recorded using indelible ink (blue ballpoint pen). The ink used must allow for easy photocopying.
- No unauthorized photocopying shall be made of any master documents. In case any photocopy of such master document is to be attached as a reference in any document, it shall be stamped as an “uncontrolled copy.” An uncontrolled document is a document that is accurate at the time it is printed but is not reviewed or updated. Usually, this document is issued to customers, regulatory authorities, or the regulatory department whenever required.
Here is a list of documents that shall meet the basic requirements of the GDP throughout the manufacturing process by all supporting teams. They include (but are not limited to):
- Batch Production Record.
- Bill of Materials (BOM)
- Protocols and reports
- Standard Operating Procedures (SOP’s)
- Working instructions
- Training assessment forms
- Certificate of Analysis (CoA) or Certificate of Compliance (CoC)
- Analytical Test Data Sheet
- Validation and technology transfer documents
- Calibration Work Books (Wherever Applicable)
- Change Control formats
General Documentation Practices:
- As described in the document preparation guide, handwritten entries shall be clear, legible, and indelible. The short and full signatures shall be documented in the records in the same manner; they have been recorded in the specimen signature register.
- Always entries shall be made in real-time (i.e., when performing an action). Attempting to record entry before or after a particular time is not a good practice and a GMP non-compliance.
- All personnel shall strive for the “Right First Time.” It means if something is done the right first time, it is done perfectly every time, and no time and money are wasted in correcting errors caused by doing it too fast or without conscious thinking and controlling quality.
- All the data shall be recorded directly on the respective document/worksheet, and no data shall be recorded on rough books / rough papers / legal pads/post-it notes.
- Entries/ observations shall be made only over issued/authorized documents.
- Original raw data shall not be discarded. Raw data means any laboratory worksheets, batch records, logbooks, or exact copies thereof that result from original observations. These records must be preserved or archived for life.
- Records shall be made or completed when each action is taken and in such a way that all significant activities concerning the manufacture of finished products are traceable.
- Entries in the records, log books, registers, or any other formats shall be done in chronological order.
- No pencils, erasers or correction pens, correction fluid, or tapes of any type shall be used for any corrections in the documents.
- All log books used for recording the data related to equipment usage and environmental monitoring shall be controlled with the date of issuance, log book number, and signed by issued personnel.
- In case of any breakdown/maintenance, the breakdown time shall be captured in the equipment usage log with a start and end time of the breakdown/maintenance.
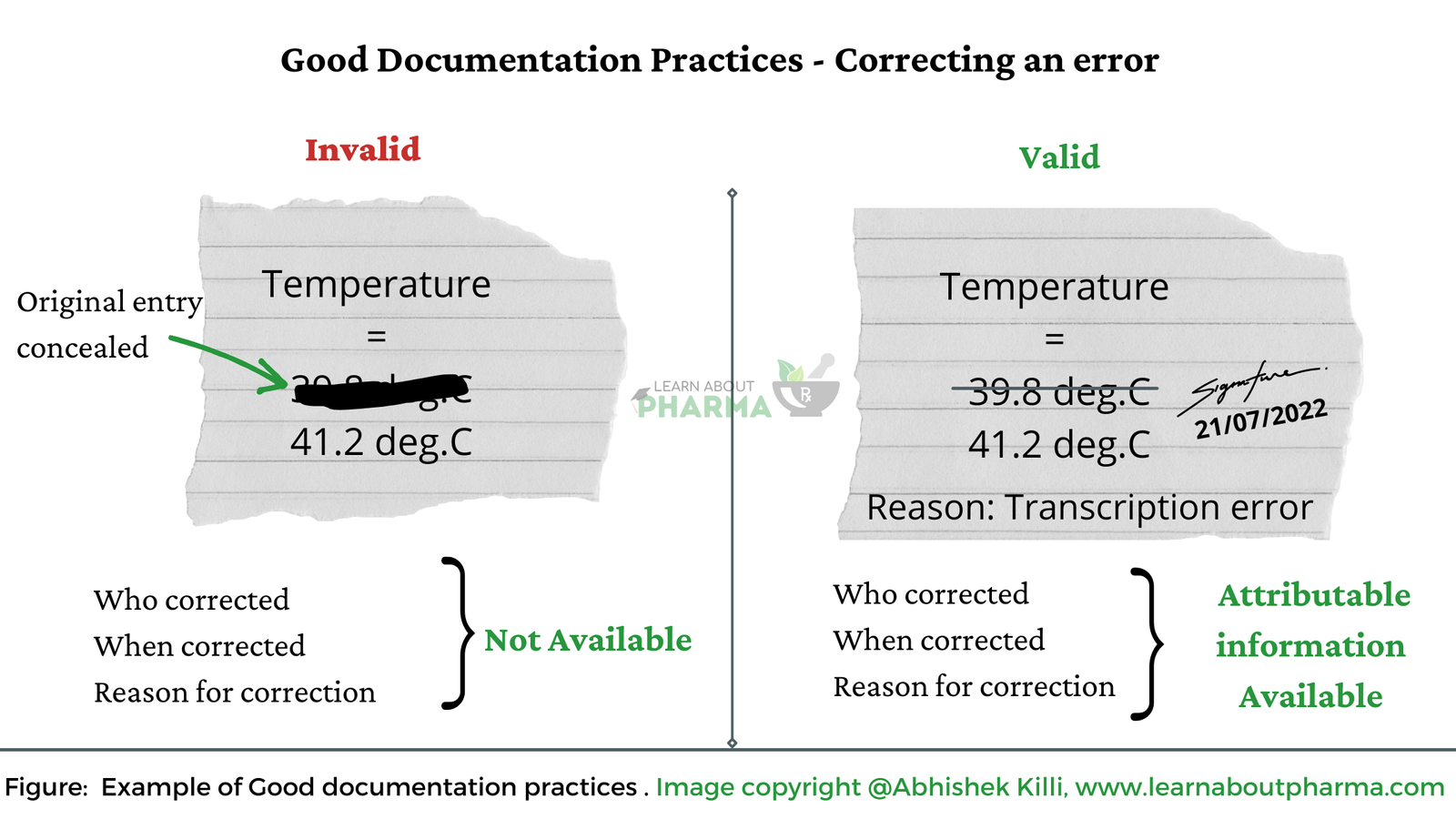
- As per GDP, Alterations made to any entry on a document shall be signed and dated; the alteration shall be visible to permit reading of the original information. Steps to perform alteration could be performed as described below:
- Cross out the wrong entry with a single line keeping the original entry clear and readable.
- Do not overwrite or scribble the wrong entry with a pen or pencil or use correction ink.
- Do the initial signature and put the date next to where the correction was made.
- Write the correct entry near the cross-out, so it is clear and legible.
- If the correction is made on a date after the date of the original entry, it must be corrected as mentioned above and countersigned and dated as on the current date.
- Write a Note /Justification explaining the reason for alteration.
- If an entire line/ paragraph/ page has to be canceled from a record such as a log book, the following steps could be followed:
- Cross it out with a line and mention the signature with the date.
- Write a note explaining the reason for crossing.
- If the correction is made on a date after the date of the original entry, it must be corrected as mentioned above and countersigned and dated on the current date.
- Do not leave any column in the record/document unfilled. If any column in a record /document is not applicable, write “NA.” If there are no comments, write Nil.
- If any page(s) is left blank, draw a line across the page and write “Cancelled / NA” across the page, and sign with a date.
- Signs indicating ditto marks (” “) shall not be used.
For Example:
Weight of X material is 2.0 kg – Valid entry
Weight ___〃___ is 2.1 kg – Invalid entry
- A second person shall review entries for accuracy and completeness. After completion of the activity, records shall be forwarded for further checking/ approval.
- The user shall retain no records in the drawers or racks after completing documents. Auditors frequently find printouts of balances, pH meters, and loose formats in the laboratory drawers, which is not considered a good practice.
- Printouts from the instruments/equipment shall be retained with the concerned document with the personnel’s date and initial/sign. Conclusions shall be drawn wherever applicable.
- Draft documents such as protocols, reports, trends, etc., used for review purposes shall be identified as “DRAFT” on the first page and disposed after accepting and incorporating all review comments in the final master copy.
- Quality assurance (QA) shall be responsible for collecting all controlled copies [photocopy] of superseded versions before issuing the current version of the document. These superseded versions shall be destroyed by shredding.
Good documentation practices (GDP)– Recording Date and Time:
- The date shall be written in any of the ways s decided by the company management: Example of date formats but not limited to:
- DD/MM/YY, i.e., 01/11/17
- DD-MM-YY, i.e., 01-11-17
- DD/MM/YYYY, i.e., 01/11/2017
- DD-MM-YYYY, i.e., 01-11-2017
- Military reporting time shall be used whenever the time is recorded in a document. For Example, 07:00 pm has to be reported as 19:00 hrs; in the case of 07:00 am, it has to be reported as 07:00 hrs.
Handling of misplaced and damaged documents:
- If any department incidentally misplaces a controlled document issued by the QA, it shall be handled through Incident and Deviation Management SOP. QA Manager shall issue a duplicate document copy after a thorough investigation.
- In case the original document is torn due to frequent handling multiple times, then each piece of the document shall be arranged and joined with clear transparent adhesive tape, and the pages shall be scanned and reviewed for legibility; content and the print of the same to be preserved with the original page/ document only, till its retention period.
- In case the original document is damaged beyond recognitiondue to mishandling or chemical spillage, then the concerned department head shall make an effort to obtain nonrecoverable entries with maximum supporting data. QA shall review, evaluate and determine the suitability of data for further action. In certain cases, incident management procedure shall be followed to find out the root cause.
Good documentation practices (GDP) –Document validation:
- Validated excel sheets shall only be used for calculations of test results. However, the excel sheets used for preparing documents for informational purposes like trends, and lists of QMS elements like Incidents, Change Control, OOS, OOT, Market complaints, etc., may not require validation.
Exceptional case in Good documentation practices (GDP):
- If any documents lack sufficient space to record observations, then a template shall be readily available to document it accordingly. This template can be a part of the concerned SOP, Document management system SOP, or any other SOP as decided suitable by the QA department.