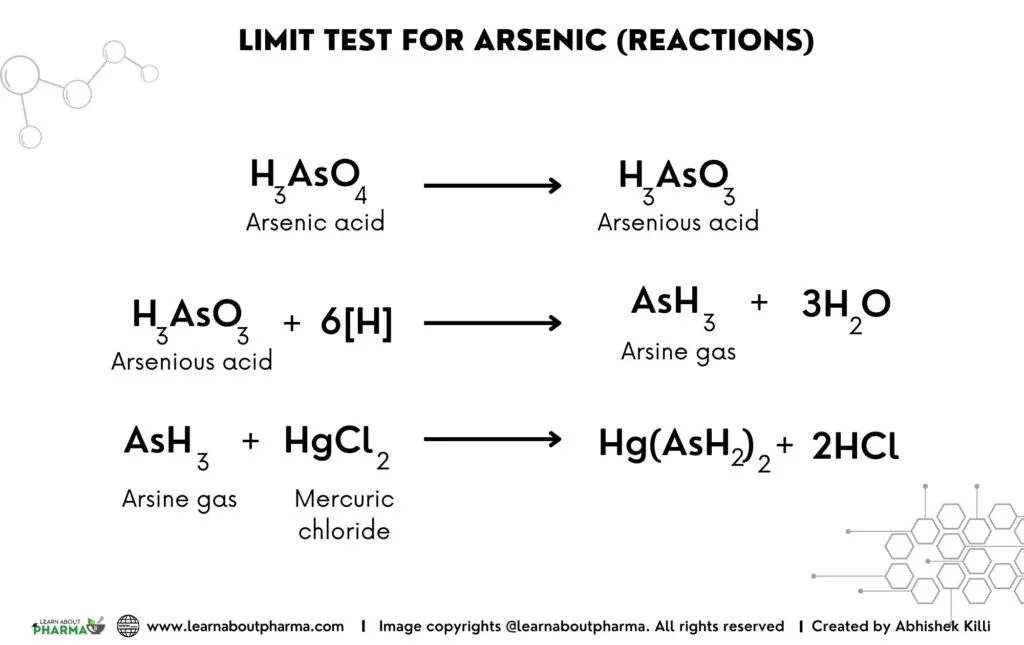
Principle of Arsenic Limit test: The limit test for Arsenic is based on the reaction of arsenic gas with hydrogen ion in the Gutzeit apparatus to form a yellow stain on mercuric chloride paper in the presence of reducing agents like potassium iodide. This reaction gives the mercuric chloride paper a dark yellow stain, and the color’s depth depends on the sample’s arsenic content. The color of the stain is finally compared with that produced from a known sample of standard arsenic solution.
- The arsenic impurity present in the sample is first converted into arsenious acid by the action of reducing agents like Zinc and HCL, potassium iodide, and stannous chloride.
- The arsenious acid is then reduced to arsine by the action of nascent hydrogen produced by the reaction between Zinc and HCL.
- The liberated arsine gas then reacts with the mercuric chloride paper to form a yellow stain.
British Pharmacopoeia (B.P) suggests using mercuric chloride paper instead of mercuric bromide paper.
You might want to read these:
Aim: This experiment aims to carry out the limit tests for Arsenic in the given samples.
Apparatus: Gutzeit apparatus, glass rod, Analytical balance and mercuric chloride paper
Chemicals: Potassium iodide, stannous chloride, HCl and Zinc
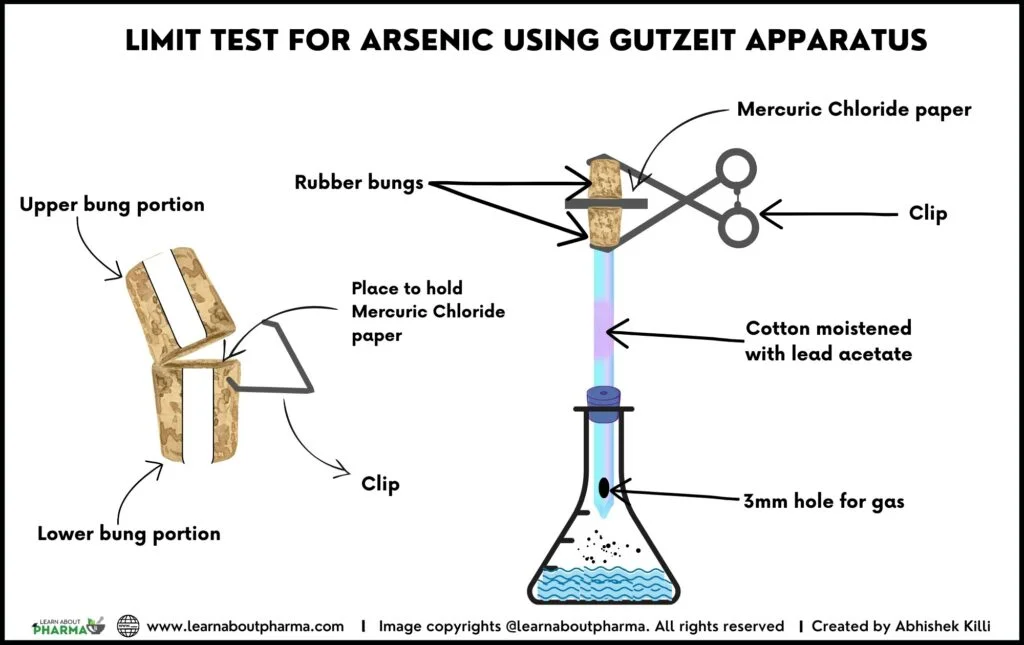
About Gutzeit Apparatus:
- The Gutzeit apparatus consists of a 120ml wide-mouthed glass bottle with a mouth of 2.5cm diameter.
- The mouth of the bottle is closed with a rubber or glass stopper which passes a glass tube of 20cm long, having an external and internal diameter of 0.8cm and 0.65cm, respectively.
- Similar to the pipette’s lower end extremity, the glass tube is constricted to about 1mm in diameter. Approximately 15mm from the tip of the lower end is a lateral orifice which should be at least 3mm below the lower surface of the stopper.
- It must be noted that when the tube is in position in the stopper, the constricted end of the tube should be above the surface of the reaction liquid in the flask, and the hole in the side is 3mm below the bottom of the bung.
- The upper end of the glass tube is cut off square and has a flat surface at right angles to the axis of the tube.
- A second glass tube of the same internal diameter as that of the first and 30 mm long, with a similar flat surface, is placed in contact with the first tube (over the top position) and is held in position by two spiral springs or clips.
- Between the two flat surfaces of the glass tubes, a disc or a small square of mercuric chloride paper is placed, which should be large enough to cover the orifice of the tube (15 mm × 15 mm).
- A loosely packed cotton is inserted into the lower glass tube previously moistened with lead acetate solution.
Procedure:
A standard and test solution is required to perform a limit test for Arsenic. Let us know the preparation of these solutions. Take two Gutzeit apparatus and name one as “test” and the other as “standard.”
Preparation of test solution:
- Accurately measure the known amount of sample as directed in the pharmacopeia and dissolve it in 50ml of distilled water.
- To this solution, add 5ml of Arsenic free 1.0M potassium iodide, 10 mL of Stannated hydrochloric acid AsT and 10 g granulated zinc AsT.
- Immediately assemble the apparatus and immerse the flask in a water bath at a temperature such that constant gas evolution is maintained for 40 minutes.
Preparation of Standard solution:
- To a known amount of standard arsenic solution in the bottle named Standard, add 50 mL of distilled water and mix it thoroughly.
- To the above solution, add 5 mL of Arsenic free 1.0 M Potassium Iodide AsT, 10 mL Stannated hydrochloric acid AsT, and 10 g granulated zinc AsT.
- Immediately assemble the apparatus and immerse the flask in a water bath maintained at a specific temperature such that constant evolution of gas is maintained for 40 minutes.
Observation:
After standing for 40 minutes, a yellow color stain is produced on the mercuric chloride paper placed on both apparatus names as test and Standard. Using the stain obtained from the bottle named Standard, compare the stain obtained on the mercuric chloride paper with that in the apparatus containing the test solution. If the stain from the test solution is no more profound than that of the standard solution, then the test sample is said to comply with the limit test for Arsenic.
Information:
- Arsenic Standard Solution (10 ppm): Dissolve 0.33 g of arsenic trioxide, previously dried at 105 deg C for 1 hour and accurately measure 5 ml of 2.0 M sodium hydroxide and dilute to 250 mL with water. Dilute 1.0 ml of this solution to 100 ml with water.
- 2.0 M Sodium Hydroxide: Dissolve 8.4 g of sodium hydroxide in sufficient carbon dioxide-free water to produce 100 ml.
- 1.0 M Potassium Iodide: Dissolve 16.6 g of potassium iodide in sufficient water to produce 100 ml.
- Stannated hydrochloric acid AsT (aka. Stannous chloride AsT): Dissolve 33 g of stannous chloride in 10 ml of hydrochloric acid and add sufficient water to produce 100 ml. Store over a little of the undissolved remaining in the solution and keep it protected from air.
- Ensure all the reagents, chemicals and water used for the test are entirely free from Arsenic.
- Carry out the test and the Standard simultaneously using an approximately similar time period
- If the stain on the mercuric chloride paper becomes darker than that of Standard, then the test shall be repeated using pure reagents. Care must be taken to protect the mercuric chloride paper from sunlight to avoid lighter or no stain.
- The suitable temperature to carry out this test is 40C.
- Cotton wool dipped in lead acetate solution is used to trap any hydrogen sulfide liberated with Arsene gas.
- Connect the rubber bung joints tightly, holding the mercuric chloride test paper so that any gas produced does not leak out.
- Care must be taken to keep the mercuric chloride paper dry.
- After completing the test, ensure that the apparatus is adequately washed with Arsenic-free HCl AsT, rinsed with water and dried.



