ICH guidelines and its references
International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is a global initiative that brings together heavy-weight regulatory authorities of Europe, Japan, the USA, and the pharmaceutical industry to prepare guidelines and discuss scientific and technical aspects of pharmaceutical product development and its registration.
The mission of the ICH is to promote public health by achieving greater harmonization through developing technical Guidelines and requirements for pharmaceutical product registration. ICH guidelines topics are divided into four major categories: Quality, Safety, Efficacy, and Multidisciplinary guidelines coded as Q, S, E, and M, respectively. Here in this article, we will list all the Quality guidelines which are the most widely followed in industrial pharmacy.
Following is the list of ICH guidelines for stability testing:
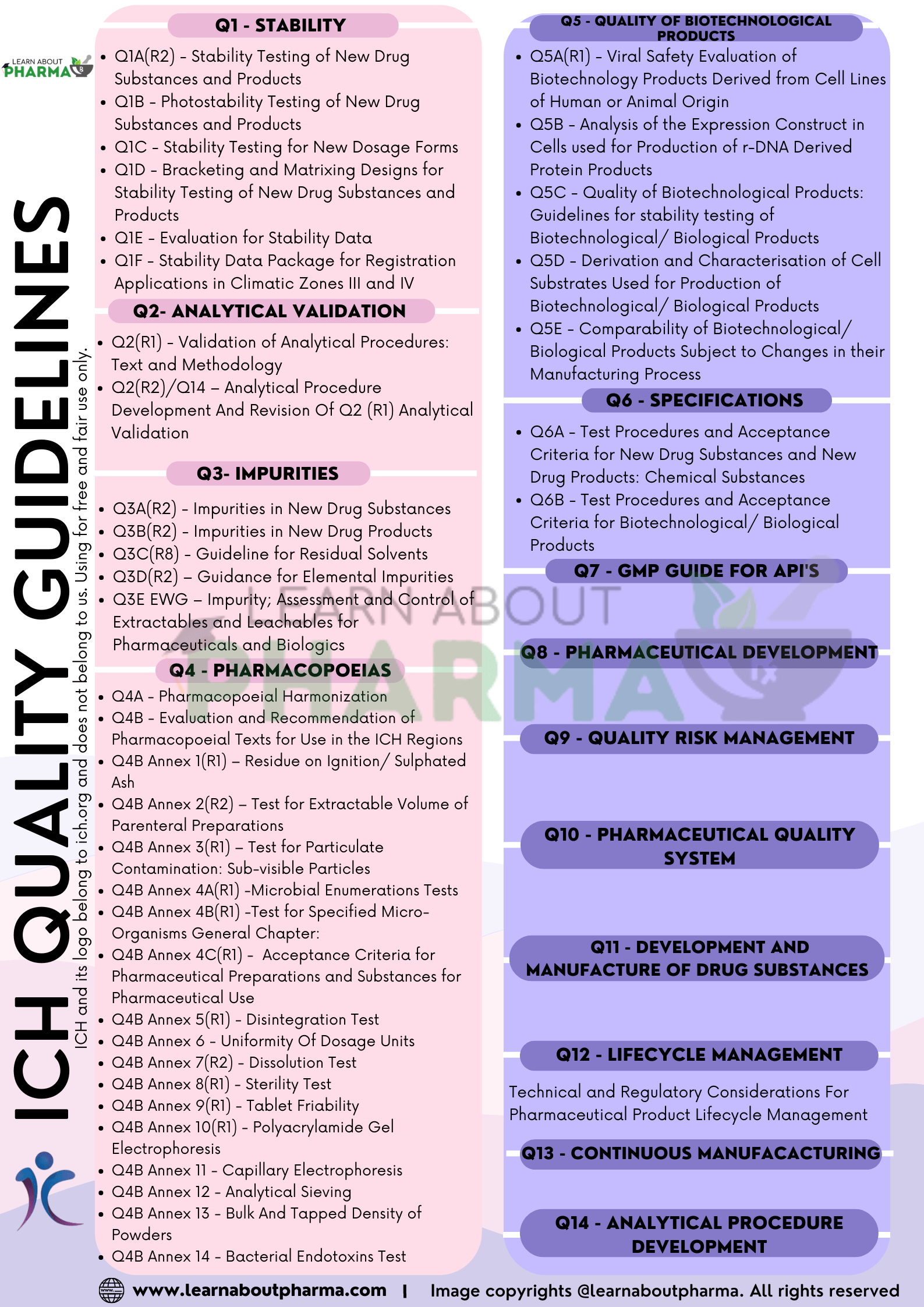
Q1 – Stability ICH guidelines
- Q1A(R2) – Stability Testing of New Drug Substances and Products: This Guideline provides recommendations on stability testing protocols, including temperature, humidity, and trial duration for Climatic Zone I and II.
- Q1B – Stability Testing: Photostability Testing of New Drug Substances and Products: his document is an annex to the main stability Guideline and gives guidance on the basic testing protocol required to evaluate the light sensitivity and stability of new drugs and products.
- Q1C – Stability Testing for New Dosage Forms: This document extends the main stability ICH guidelines for new formulations of already approved medicines and defines the circumstances under which reduced stability data can be accepted.
- Q1D – Bracketing and Matrixing Designs for Stability Testing of New Drug Substances and Products: This document is intended to address recommendations on the application of bracketing and matrixing to stability studies conducted in accordance with principles outlined in the main stability Guideline.
- Q1E – Evaluation for Stability Data: This document extends the main stability Guideline by explaining possible situations where extrapolation of retest periods or shelf-lives beyond the real-time data may be appropriate. Furthermore, it provides examples of statistical approaches to stability data analysis.
- Q1F – Stability Data Package for Registration Applications in Climatic Zones III and IV: This document was withdrawn in 2006 and decided to leave the definitions of storage conditions in Climatic Zones III and IV to the respective regions and WHO.
Q2 – Analytical Validation ICH guidelines:
- Q2(R1) – Validation of Analytical Procedures: Text and Methodology: This document identifies the validation parameters for various analytical methods. It also discusses the characteristics that must be considered during the validation of the analytical procedures, which are included in registration applications.
- Q2(R2)/Q14 – Analytical Procedure Development And Revision Of Q2 (R1) Analytical Validation: This guideline presents a discussion of elements for consideration during the validation of 2 analytical procedures included as part of registration applications submitted within the ICH 3 member regulatory authorities. Q2(R2) provides guidance and recommendations on how to 4 derive and evaluate the various validation tests for each analytical procedure. This guideline 5 serves as a collection of terms and their definitions.
Q3E – Impurities ICH guidelines:
- Q3A(R2) – Impurities in New Drug Substances: This ICH guidelines addresses the chemistry and safety aspects of impurities, including the listing of impurities in specifications, and defines the thresholds for reporting, identification, and qualification.
- Q3B(R2) – Impurities in New Drug Products: This guidance complements the guideline on impurities in new drug substances and provides advice in regard to impurities in products containing new, chemically synthesized drug substances.
- Q3C(R8) – Guideline for Residual Solvents: This guidance provides recommendations on using less toxic solvents in manufacturing drug substances and dosage forms and setting pharmaceutical limits for residual solvents (volatile organic impurities) in drug products.
- Q3C(R9) – Maintenance EWG; Maintenance of the guideline for residual solvents
- Q3D(R2) – Guidance for Elemental Impurities: This guideline is for the control of elemental impurities in new drug products (medicinal products), and it establishes Permitted Daily Exposures (PDEs) for 24 Elemental Impurities (EIs) for drug products administered by the oral, parenteral and inhalation routes of administration.
- Q3D(R3) – Maintenance EWG; Maintenance of the guideline for Elemental Impurities
- Q3D Training; Implementation of Guidance for Elemental Impurities: This document includes a comprehensive training program with supporting documentation for effective implementation.
- Q3E EWG – Impurity; Assessment and Control of Extractables and Leachables for Pharmaceuticals and Biologics: This guidance was established to work on the development of the Q3E guideline on the assessment and control of extractable and leachable (E&L) and is expected would assist both applicants and regulators by providing a focus on critical aspects, and improving transparency in requirements for medicinal products including drug delivery device components.
Q4 – Pharmacopoeias ICH guidelines:
- Q4A – Pharmacopoeial Harmonization: This document details the harmonization of major and widely followed pharmacopeias like USP, JP, and EP.
- Q4B – Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH Regions: This document describes a process for the evaluation and recommendation by the Q4B Expert Working Group (EWG) of selected pharmacopoeial texts to facilitate their recognition by regulatory authorities for use as interchangeable in the ICH regions
- Q4B Annex 1(R1) – Residue on Ignition/Sulphated Ash General Chapter: This annex results from the Q4B process for Residue on Ignition/Sulphated Ash General Chapter.
- Q4B Annex 2(R2) – Test for Extractable Volume of Parenteral Preparations General Chapter: This annex is the result of the Q4B process for the Test for Extractable Volume of Parenteral Preparations General Chapter.
- Q4B Annex 3(R1) – Test for Particulate Contamination: Sub-visible Particles General Chapter: This annex results from the Q4B process for Test for Particulate Contamination.
- Q4B Annex 4A(R1) – Microbiological Examination of Non-Sterile Products: Microbial Enumerations Tests:
- Q4B Annex 4B(R1) – Microbiological Examination of Non-Sterile Products: Test for Specified Micro-Organisms General Chapter:
- Q4B Annex 4C(R1) – Microbiological Examination of Non-Sterile Products: Acceptance Criteria for Pharmaceutical Preparations and Substances for Pharmaceutical Use General Chapter:
- Q4B Annex 5(R1) – Evaluation and Recommendation of Pharmacopoeial Texts For Use In The Ich Regions On Disintegration Test General Chapter
- Q4B Annex 6 – Evaluation and Recommendation of Pharmacopoeial Texts For Use In The ICH Regions On Uniformity Of Dosage Units General Chapter
- Q4B Annex 7(R2) – Evaluation and Recommendation of Pharmacopoeial Texts For Use In The Ich Regions On Dissolution Test General Chapter
- Q4B Annex 8(R1) – Evaluation and Recommendation of Pharmacopoeial Texts For Use In The ICH Regions On Sterility Test General Chapter
- Q4B Annex 9(R1) – Evaluation and Recommendation of Pharmacopoeial Texts For Use In The ICH Regions On Tablet Friability General Chapter
- Q4B Annex 10(R1) – Evaluation and Recommendation of Pharmacopoeial Texts For Use In The ICH Regions On Polyacrylamide Gel Electrophoresis General Chapter
- Q4B Annex 11 – Evaluation and Recommendation of Pharmacopoeial Texts For Use In The ICH Regions On Capillary Electrophoresis General Chapter
- Q4B Annex 12 – Evaluation and Recommendation of Pharmacopoeial Texts For Use In The ICH Regions On Analytical Sieving General Chapter
- Q4B Annex 13 – Evaluation and Recommendation of Pharmacopoeial Texts For Use In The ICH Regions On Bulk Density And Tapped Density of Powders General Chapter
- Q4B Annex 14 – Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH Regions on Bacterial Endotoxins Test
- Q4B FAQs – Evaluation and Recommendation of Pharmacopoeial Texts for Use in the ICH Regions
Q5: Quality of Biotechnological Products ICH guidelines
- Q5A(R1) – Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin: This document is concerned with testing and evaluation of the viral safety of biotechnology products derived from characterized cell lines of human or animal origin and outlines data that should be submitted in the marketing application/registration package.
- Q5B – Analysis of the Expression Construct in Cells used for Production of r-DNA Derived Protein Products: This document is intended to describe the types of information that are considered valuable in assessing the structure of the expression construct used to produce recombinant DNA-derived proteins.
- Q5C – Quality of Biotechnological Products: Guidelines for stability testing of Biotechnological/ Biological Products: This document augments the stability Guideline (Q1A) and deals with the particular aspects of stability test procedures needed to take account of the unique characteristics of products in which the active components are typically proteins and/or polypeptides.
- Q5D – Derivation and Characterisation of Cell Substrates Used for Production of Biotechnological/ Biological Products: This document provides broad guidance on appropriate standards for the derivation of human and animal cell lines and microbial cells used to prepare biotechnological/biological products and for the preparation and characterization of cell banks to be used for production
- Q5E – Comparability of Biotechnological/ Biological Products Subject to Changes in their Manufacturing Process: This document aims to provide principles for assessing the comparability of biotechnological/biological products before and after changes are made in the manufacturing process for the drug substance or drug product.
Q6 – Specifications ICH guidelines:
- Q6A – Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances: This document provides guidance on the setting and justification of acceptance criteria and the selection of test procedures for new drug substances of synthetic chemical origin and new drug products produced from them, which have not been registered previously in the ICH regions.
- Q6B – Test Procedures and Acceptance Criteria for Biotechnological/ Biological Products: This document provides general principles on the setting and justification of a uniform set of international specifications for proteins and polypeptides which are produced from recombinant or non-recombinant cell-culture expression systems.
Q7 – Good Manufacturing Practice Guide for APIs (Active Pharmaceutical Ingredients) ICH guidelines:
This ICH guidelines is intended to provide guidance regarding Good Manufacturing Practice (GMP) for the manufacturing of Active Pharmaceutical Ingredients (APIs) under an appropriate system for managing quality. It is also intended to help ensure that APIs meet the requirements for quality and purity that they purport or are represented to possess.
Q8 – Pharmaceutical Development ICH guidelines:
Q8(R2) – Pharmaceutical Development: These ICH guidelines is intended to provide guidance on the contents of Section 3.2.P.2 (Pharmaceutical Development) for drug products as defined in the scope of Module 3 of the Common Technical Document (ICH topic M4). The guideline does not apply to the contents of submissions for drug products during the clinical research stages of drug development. However, the principles in this guideline are essential to consider during these stages.
Q9 – Quality Risk Management ICH guidelines: These ICH guidelines provides principles and examples of tools for quality risk management that can be applied to different aspects of pharmaceutical quality. These aspects include development, manufacturing, distribution, and the inspection and submission/review processes throughout the lifecycle of drug substances, drug (medicinal) products, biological and biotechnological products (including the use of raw materials, solvents, excipients, packaging and labeling materials in drug (medicinal) products, biological and biotechnological products).
Q10 – Pharmaceutical Quality System ICH guidelines: This Guideline applies to the systems supporting the development and manufacture of pharmaceutical drug substances and drug products, including biotechnology and biological products, throughout the product lifecycle.
Q11 – Development and Manufacture of Drug Substances (Chemical Entities and Biotechnological/ Biological Entities): This Guideline describes approaches to developing and understanding the manufacturing process of the drug substance and also provides guidance on what information should be provided in Module 3 of the Common Technical Document (CTD) Sections 3.2.S.2.2 – 3.2.S.2.6 (ICH M4Q).
Q12 – Lifecycle Management ICH guidelines:
Q12 – Technical and Regulatory Considerations For Pharmaceutical Product Lifecycle Management: These new ICH guidelines are proposed to provide a framework to facilitate the management of post-approval Chemistry, Manufacturing, and Controls (CMC) changes in a more predictable and efficient manner across the product lifecycle.
Q13 – Continuous Manufacturing of Drug Substances and Drug Products: These new ICH guidelines are proposed to Capture critical technical and regulatory considerations; Allow drug manufacturers to employ flexible approaches to develop, implement, or integrate CM for the manufacture; Provide guidance to industry and regulatory agencies regarding regulatory expectations on the development, implementation, and assessment of CM technologies
Q14 – Analytical Procedure Development
References:
- ICH Quality guidelines (https://www.ich.org/page/quality-guidelines)