Definition:
Dissociation is breaking up large complex chemical molecules into smaller pieces. When this occurs with an acid or base, Dissociation constant is an equilibrium constant that measures the tendency of large molecules/compounds dissociate or separate reversibly into smaller components like a conjugate base and free hydrogen ions. For example: Splitting up of common salt into its component ions. A dissociation constant depicts the concentration of conjugate bases and free hydrogen ions (Products) to that of the original acid compounds (reactant).
An Explanation for Dissociation Constant:
In terms of acids and bases, dissociation is a process where acids or base separates into ions in water. The strength of an acid is determined by the moles of H3O+ ions produced from each mole of acid that dissociates. And the same goes with a base strength determined by the moles of OH– produced from each mole of the base that dissolves in water. Higher the dissociation, the strong the acid or base is. Strong acid and base dissociate entirely in water, whereas weak acids and weak bases slightly dissociate, leaving most of the initial acid undissociated. Because weak acids in water dissociate only slightly, the ion products reach equilibrium with the undissociated weak acid molecules. For example, when a weak acid (HA) is added to water, an equilibrium is formed where HA protonate the H2O to form Hydronium ion (H3O+) and the conjugate base (A–)
HA(aq) + H2O(l) ⇋ H3O+(aq) + A– (aq)
In the case of a base a similar equilibrium exists. The base will accept a proton from water and form a conjugate acid, BH+:
HB(aq) + H2O(l) ⇋ BH+(aq) + OH–(aq)
Formula to find Dissociation Constant:
Typically, to determine the dissociation constant of an acid or base, we need to measure how much of a base or acid is dissociated when came in contact with water. As discussed above, a conjugate base of an acid and hydronium ions are formed when an acid protonates the water. During this protonation process, acid molecules donate H+ ions to H2O to form Hydronium (H3O+) ions and conjugate base (A–). The reverse reaction also occurs here, where hydronium ions release a hydrogen atom and transfer it back to acid. This movement of hydrogen ions from acid to water and vice versa continues until an equilibrium is reached where the products and reactants are exchanged.
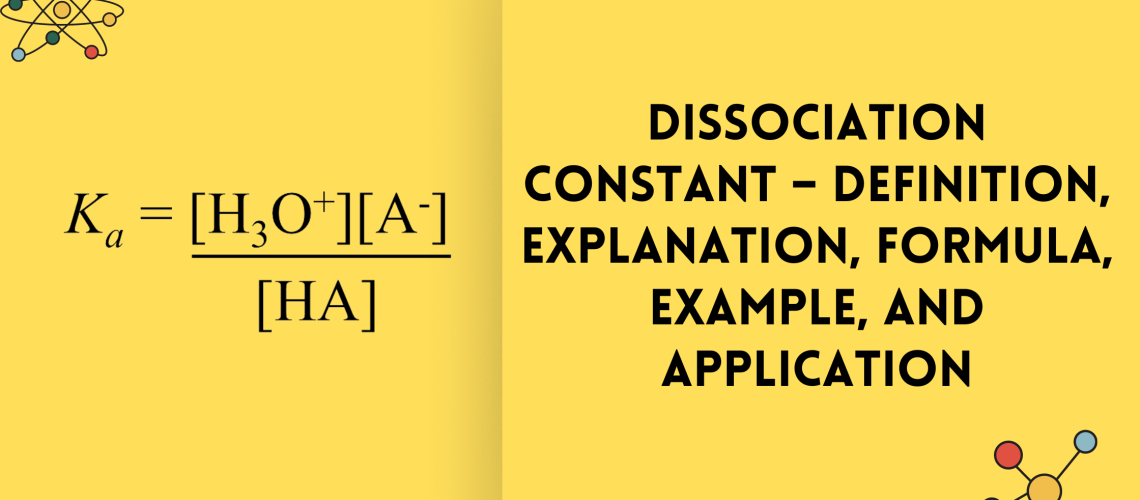
An acid dissociation expression, Ka, can be written for weak acids that give the ratio of the concentrations of products to the weak acid reactants. Because the equilibrium is used for calculating the concentrations of weak acids, very little water reacts. The concentration of water during the reaction is, therefore, a constant and can be excluded from the expression for K. This gives rise to a special equilibrium constant, Ka, known as the acid dissociation constant. For example, the acid dissociation expression for the equilibrium equation of acid shown in the explanation above is written.
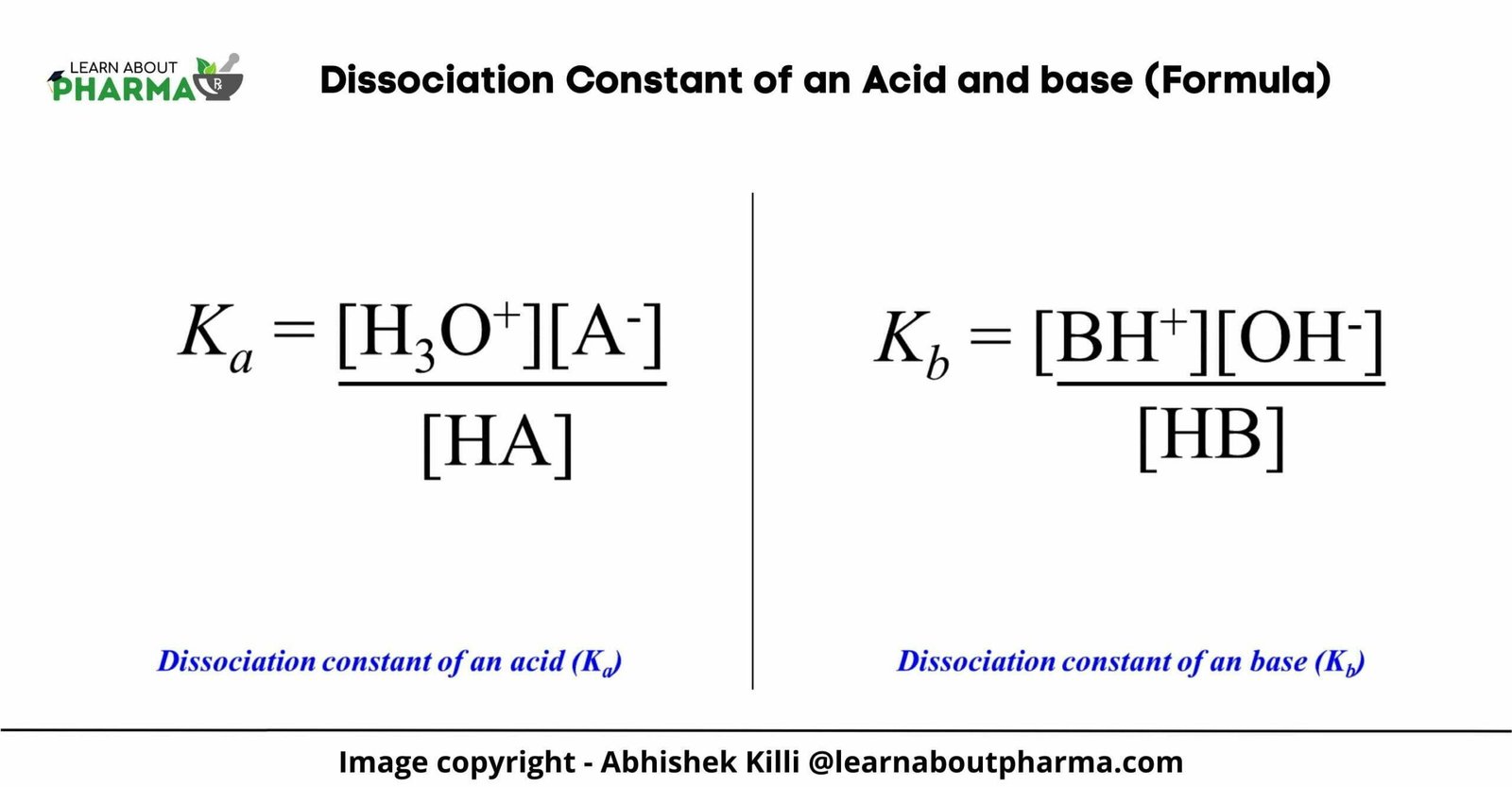
Ka = [H3O+][A-] / [HA]
Similarly, the base dissociation constant expression for the equilibrium equation of base shown in the explanation above is written.
Kb = [BH+][OH–] / [HB]
In the above equations of acid, the numerator denotes the concentration of free hydronium ions multiplied by the conjugate base concentration. At the same time, the numerator is the concentration of the initial acid substance. Most of the time, the concentration of the numerator values is unknown. Since the equilibrium dissociation constant requires these numbers, they must be calculated before proceeding. These can be calculated by testing the pH of the solution using pH meter. Once the pH is obtained, the number of free hydrogen ions can be calculated using the following equation:
[H] = 10-pH
Remember that H+ can be used to replace H3O+, thus simplifying the depiction of the reaction between a weak acid with water and its expression is written as
HA(aq) ⇋ H+(aq) + A– (aq)
A logarithmic measure of Ka is the more common and convenient term used in practice. pKa is the negative log of Ka and is also referred to as acid dissociation constant.
Example:
Let us assume we prepared a 0.1M Formic acid solution in water and for which we want to determine the acid dissociation constant. In this case, the formic acid equilibrium equation can be written as below:
HCHO2 (aq) + H2O(l) ⇋ H3O+(aq) + AHO2– (aq)
To find out the H+ ion concentration of the solution, we need to find the pH of the above solution. The pH is tested and found to be 2.38. Now using this value, we want to first convert the pH of the solution into the concentration of hydrogen ions. This is done by the following:
[H] = 10– 2.38
[H] = 0.00412 mol/L
Now, referring back to the equilibrium equation of formic acid, it is observed that the concentration of hydrogen ions and conjugate base are equal at equilibrium. We know this because dissociating a single formic acid molecule yields one hydrogen ion and one conjugate base. The concentration of hydrogen ions we calculated with the pH equation is also the conjugate base concentration. With these values, we want to calculate the acid dissociation constant:
Ka = [0.00412] [0.00412] / [0.1]
Ka = 0.000177 = 1.77 x 10-5
Applications of Dissociation Constant:
- The concept is applied in the fields of Pharmacology and Chemistry. It determines the affinity between protein and a ligand in protein-ligand binding. A higher dissociation constant indicates a less tightly bound ligand.
- pKa values of proteins and amino acid side chains are of significant importance to finding the activity of enzymes and the stability of proteins
- Frequently applied in environmental science to find out the acid-base equilibria of lakes and rivers.
- Ka of a drug can be helpful to determine its solubility, side of protein binding and rate of absorption in the gastrointestinal tract. Weakly acidic drugs are less ionized, readily available in the stomach and hence have a higher absorption rate. In the same way, drugs which are weak bases are readily absorbed in the intestines.
References:
- https://study.com/academy/lesson/what-is-the-dissociation-constant.html
- https://preparatorychemistry.com/Bishop_weak_acid_Equilibrium.html
- https://www.pearson.com/content/dam/one-dot-com/one-dot-com/us/en/higher-ed/en/products-services/course-products/timberlake-basic-chem-5e-info/pdf/timberlake-9780134074306-chap14.pd