Reverse phase chromatography (RPC) is a widely used liquid HPLC technique that separates molecules based on hydrophobic interactions. This process involves solute molecules in the mobile phase interacting with alkyl chain ligands covalently bonded to the stationary phase particles. As a result, the stationary phase, characterized by its hydrophobic nature, exhibits a stronger affinity for less polar substances, effectively separating molecules according to their hydrophobicity.
Often referred to as adsorption chromatography, reverse phase chromatography operates primarily through a partitioning mechanism. This method is pivotal in both analytical and preparative applications, particularly in the biological domain for the separation and purification of complex compounds. Reverse phase chromatography is especially effective in separating molecules such as proteins, peptides, and nucleic acids, achieving high recovery and resolution. This efficacy stems from the reversible adsorption of solute molecules, which vary in their levels of hydrophobicity.
In RPC, a nonpolar stationary phase and a polar mobile phase are used—a configuration that is inverse to traditional chromatographic techniques. This inversion in the phase properties is why the method is termed “reverse phase chromatography”.
Principle of reverse phase chromatography:
The separation mechanism in RPC is fundamentally governed by the hydrophobic binding interactions between the solute molecules in the mobile phase and the immobilized hydrophobic ligands that constitute the stationary phase. The distribution of solute molecules between these two phases is crucial to the separation process.
This distribution is influenced by several factors:
- The binding properties of the medium
- The hydrophobicity of the solute
- The composition of the mobile phase
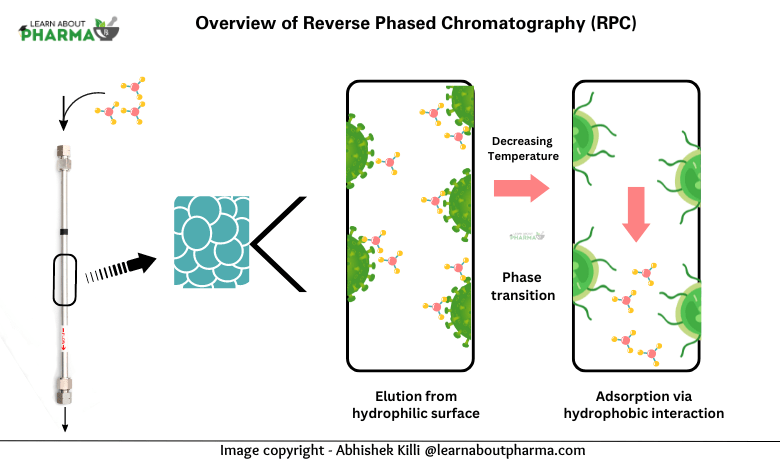
In reverse phase chromatography, the experimental setup is designed to initially favor the adsorption of solute molecules from the mobile phase onto the stationary phase. This preference enables the effective separation of compounds based on their hydrophobic characteristics, facilitating a detailed analysis or purification of the sample.
Matrix used in Reverse Phase Chromatography:
The matrix in RPC plays a critical role in the chromatographic process, requiring both chemical and structural stability to function effectively. Common materials used for the reverse phase chromatography matrix include silica and synthetic polystyrene, both of which meet these stability requirements.
Matrix Base and Stability
Silica is favored for its large surface area, allowing a substantial number of hydrophobic ligands to be chemically bonded, thus offering high solute loading capacities. Polystyrene, a synthetic organic polymer, is noted for its exceptional chemical stability, particularly in extreme acidic or basic conditions. This makes it suitable for separations that need to occur outside the neutral pH range, with polystyrene media remaining stable across pH levels from 1 to 12.
Particle Size and Separation Scale
The particle size of the matrix beads is selected based on the separation’s scale and precision requirements. Larger beads (over 10 μm in diameter) are typically used in large-scale preparative processes due to their higher capacities and lower pressure requirements. Conversely, smaller beads (3–5 μm in diameter) are preferable for small-scale preparative and analytical tasks, providing finer resolution.
Ligand Characteristics and Selection
The hydrophobicity of the ligands attached to the matrix is crucial and is typically inversely related to the hydrophobicity of the target molecules. For instance, C18 ligands, which are long-chain hydrophobic ligands, are ideal for separating chemically synthesized peptides and oligonucleotides. C8 ligands, being slightly less hydrophobic, are better suited for proteins and recombinant peptides. The ligand density, bonding chemistry, and the pore size of the beads are also key factors that affect the efficacy of the separation process.
Functionalization and Chain Length
The stationary phases in reverse phase chromatography are often monomeric, where the silica support is functionalized with a silanizing reagent that has one hydrolyzable group. The chain length, referring to the total number of carbon atoms in the ligands, and their bonding density primarily determine the phase’s hydrophobicity.
Mobile Phase Composition in Reverse Phase Chromatography:
The mobile phase in reverse phase chromatography plays a pivotal role in the separation process, largely determining the interaction between solutes and the stationary phase. This phase typically consists of a blend of water with one or more miscible organic solvents, such as acetonitrile, methanol, ethanol, tetrahydrofuran, and isopropanol.
Composition and Purpose
In most Reverse phase chromatography applications, binary mixtures of water and an organic solvent are utilized. However, to enhance the strength and selectivity of the elution, ternary or quaternary mixtures incorporating two or three different organic solvents may also be used. The organic solvents serve to adjust the mobile phase’s polarity—keeping it low enough to dissolve partially hydrophobic solutes yet high enough to facilitate effective binding to the hydrophobic stationary phase.
Use of Additives
Buffers and other additives, such as ion-pairing agents like trifluoroacetic acid, may be incorporated into the mobile phase. These additives help modify the phase behavior, improving the separation efficiency and enabling precise control over the solute-stationary phase interactions. Ion-pairing agents, for instance, enhance the retention of charged solutes, providing a mechanism by which molecules of interest bind to and dissociate from the column matrix.
Gradient Elution
A key strategy in reverse phase chromatography is gradient elution, where the concentration of the organic solvent in the mobile phase is varied over time. This method adjusts the mobile phase polarity dynamically, allowing for the selective elution of molecules based on their hydrophobic properties. By increasing the organic solvent concentration, the polarity is decreased, facilitating the dissociation of the bound molecule from the stationary phase and thus achieving effective separation.
Detailed procedure for Reverse Phase chromatography:
Based on the hydrophobic interactions of components of a sample mixture with the stationary phase, several key steps are involved in RPC to effectively separate and analyze. Here’s a typical workflow:
- Preparation of the Mobile Phase: The mobile phase usually consists of a mixture of water and organic solvents like acetonitrile or methanol. The proportion of organic solvent can vary depending on the desired strength and the hydrophobicity of the analytes.
- Sample Preparation: The sample is prepared by dissolving it in a solvent that is compatible with the mobile phase to ensure good solubility and optimal interaction with the stationary phase.
- Column Selection and Preparation: A suitable reverse-phase column (typically packed with hydrophobic C18 bonded silica particles) is chosen. The column needs to be equilibrated with the initial mobile phase before injection to ensure reproducibility and consistent performance.
- Injection of the Sample: A small volume of the prepared sample is injected into the chromatographic system. The injection must be made in a solvent that is compatible with the mobile phase to avoid precipitation or shock to the column.
- Separation: As the mobile phase carries the sample through the column, components of the mixture interact differently with the hydrophobic stationary phase. Non-polar compounds retain longer due to stronger hydrophobic interactions, while more polar compounds elute earlier.
- Detection: As compounds elute from the column, they pass through a detector (commonly UV, fluorescence, or mass spectrometry). The detector generates a signal proportional to the concentration of each component, resulting in a chromatogram.
- Data Analysis: The chromatogram is analyzed to determine the retention times and the area under the peaks, which helps in identifying and quantifying the components of the sample.
- Column Regeneration: After the run, the column is typically washed with a stronger organic solvent to remove any residual compounds and re-equilibrated with the starting mobile phase.
- Validation and Repetition: The process may be repeated for validation or to refine separation conditions to achieve better resolution or faster analysis times.
These steps outline the general process of conducting a reverse phase chromatographic analysis, which is widely used for the separation and analysis of complex mixtures in pharmaceutical, biological, and environmental samples.
Practical Applications of Reverse Phase Chromatography:
Reverse phase chromatography stands as a cornerstone in the field of analytical chemistry, especially for the separation and analysis of biomolecules such as peptides, proteins, and nucleotides. This method is preferred for numerous reasons:
- Matrix Stability and Versatility: Reverse phase chromatography is celebrated for its stable matrix performance across a diverse array of mobile phase conditions. This stability supports the method’s high versatility, making reversed-phase sorbents preferred over normal-phase sorbents. Today, they are employed in over 80% of all analytical chromatographic separations.
- Reproducibility and High Resolution: The technique ensures reproducible outcomes, which is critical for consistent analytical results. Furthermore, it allows for excellent resolution of target molecules, whether they are closely related or structurally distinct, facilitating detailed study and characterization.
- Efficiency and Throughput: RP-HPLC is known for its appreciable recoveries and high throughput capabilities. These features make it an efficient choice for both analytical and preparative applications, spanning the separation of synthetic and biological molecules.
- Ease of Separations with Gradient Elution: Gradient elution, a process where the composition of the mobile phase is progressively changed during the separation, simplifies the process and enhances the effectiveness of separations. This technique is particularly beneficial when dealing with complex samples.
- Challenges with Large Proteins: Despite its widespread success, RP-HPLC faces challenges when separating larger polypeptides (greater than 10 kDa) and globular proteins. Problems such as loss of enzymatic activity and poor yields due to protein denaturation during the separation process are notable limitations of the technique in handling large proteins.
Factors Influencing Separation in Reverse Phase Chromatography::
Reversed phase chromatography (RPC) is influenced by various factors that determine the efficiency and effectiveness of the separation process. These factors are critical in tailoring the reverse phase chromatography technique to specific analytical needs:
Column Length
- Large Biomolecules: The resolution of large biomolecules such as proteins, large peptides, and nucleic acids is generally less dependent on column length. These molecules can be effectively purified on shorter columns without a significant impact on resolution.
- Small Molecules: Conversely, the resolution of smaller organic molecules and peptides can be enhanced by increasing the column length, although the effect varies.
- Mobile Phase Composition
- Buffering Capacity: Often referred to informally as “buffers,” mobile phases in reverse phase chromatography typically include strong acids at low pH levels and high concentrations of organic solvents. It’s crucial to maintain sufficient buffering capacity, especially when operating near physiological conditions.
- Sensitivity to Composition: The elution of large molecules is highly sensitive to small changes in the concentration of organic modifiers, due to the specific partition coefficients of high molecular weight solutes.
- Organic Solvents
- Polarity Reduction: The addition of organic solvents to the aqueous mobile phase decreases its polarity, thus increasing the eluting strength of the mobile phase. Common organic modifiers used include acetonitrile, isopropanol, and methanol, all of which are largely UV transparent—a key characteristic since UV detectors commonly monitor column elution.
- Ion Suppression
- Effect of pH: The pH of the mobile phase can significantly affect the retention of proteins and peptides, which possess ionizable groups. Strong acids such as trifluoroacetic acid or ortho-phosphoric acid are typically used to keep the pH low, preventing the ionization of acidic groups within solute molecules, thus impacting retention behavior.
- Ion Pairing Agents
- Modification of Retention: Ion pairing agents added to the mobile phase can alter the retention times of solutes like proteins, peptides, and oligonucleotides. These agents bind to solutes through ionic interactions, changing the hydrophobicity of the solute and thus influencing its retention characteristics.
Advantages of Reversed Phase Chromatography
Reversed-phase chromatography offers several key benefits that make it a preferred method in various analytical contexts:
- Cost-Effectiveness and Efficiency: RPC is a relatively affordable technique compared to other chromatographic methods. It delivers accurate results with minimal sample requirements, making it efficient and resource-effective.
- pH Selectivity: The method allows for the use of pH selectivity to enhance separation capabilities. This feature is particularly useful for manipulating the elution order of compounds and improving the resolution of complex mixtures.
- Versatility in Compound Analysis: Reverse phase chromatography is capable of analyzing a broad spectrum of compounds. This includes neutral, polar, and nonpolar solutes, as well as acidic, basic, and amphoteric substances, making it highly versatile.
- Retention of Organic Molecules: Most organic molecules are effectively retained by the hydrophobic stationary phase in RPC, which facilitates their separation and analysis.
Disadvantages of Reversed Phase Chromatography
Despite its numerous advantages, reverse phase chromatography also has some limitations that may affect its application:
- Pressure Requirements: Reversed phase chromatography (RP-HPLC) requires the generation of high pressure, which can pose technical challenges and increase system wear and tear.
- Limitations with Certain Compounds: RPC can be challenging when analyzing amines and compounds that are insoluble in water. This restricts its utility with certain types of samples.
- Need for Supplementary Methods: To confirm the identity of analytes, additional analytical methods are often necessary, complicating the analytical process and increasing time and cost.
- Technical Expertise: Effective use of RPC demands more technical knowledge and skills. This requirement for specialized expertise can be a barrier in settings where training and resources are limited.
Troubleshooting Common Issues in Reverse Phase Chromatography:
Troubleshooting in reverse phase chromatography often revolves around addressing issues with peak shapes, retention times, and reproducibility. Here are three common troubleshooting scenarios and their possible solutions:
Poor Peak Shape (Tailing or Fronting Peaks)
Potential Causes:
- Overloading of the sample onto the column.
- Interaction between analytes and active sites on the column.
- Suboptimal pH of the mobile phase affecting the analyte’s interaction with the stationary phase.
Solutions:
- Reduce the sample size or concentration.
- Add a buffer to the mobile phase to control the pH and minimize interactions.
- Consider using a column with a different bonding or endcapping to reduce interactions with residual silanol groups.
Decreased Retention Time or Inconsistent Retention Times
Potential Causes:
- Changes in the composition or the flow rate of the mobile phase.
- Column degradation or changes in column temperature.
- Contamination or deterioration of the stationary phase.
Solutions:
- Check and adjust the mobile phase composition and ensure consistent flow rates.
- Maintain a consistent column temperature during runs.
- Clean the column with a strong solvent or replace the column if it has degraded significantly.
Low Resolution Between Peaks
Potential Causes:
- Inadequate selectivity of the stationary phase for the analytes.
- Suboptimal mobile phase composition.
- Insufficient column efficiency due to packing issues or column age.
Solutions:
- Experiment with different mobile phase compositions, including adjusting the water/organic solvent ratio or adding modifiers.
- Try a different column that offers better selectivity for the compounds of interest.
- Replace the column if it is old or the packing has degraded, which can be indicated by broad, inefficient peaks.
Each of these scenarios requires a methodical approach to identify the specific cause and apply the appropriate solution to ensure optimal performance of the Reverse phase chromatography system



